Key Takeaways:
I. Liquid biopsy offers a less invasive, more convenient alternative to traditional tissue biopsies, potentially improving patient adherence and enabling earlier cancer detection.
II. Real-time treatment monitoring and early detection of drug resistance through liquid biopsy can lead to more effective personalized therapies and improved patient outcomes.
III. The liquid biopsy market is experiencing rapid growth and consolidation, with pharmaceutical companies actively acquiring key players to gain a competitive edge in this emerging field.
Pharmaceutical giants and diagnostic testing leaders are actively seeking to acquire high-momentum liquid biopsy startups, signaling a major shift in the cancer diagnostics landscape. As pharma companies double down on precision oncology, liquid biopsies—which offer real-time, non-invasive tumor profiling through a simple blood draw—are poised to revolutionize cancer care. These tests promise to significantly improve patient adherence, which currently sits as low as 60% for some common cancer screenings like colorectal cancer. Moreover, liquid biopsies offer the ability to dynamically track treatment response, detect drug resistance early, and adjust therapy accordingly, potentially improving outcomes while reducing the costs associated with failed treatments. This article explores the key players, technological advancements, and market dynamics driving this rapid expansion, offering insights for investors, researchers, and clinicians alike.
Understanding Liquid Biopsy Technology
Liquid biopsy leverages the analysis of various biomarkers found in bodily fluids, primarily blood, to detect and monitor cancer. Circulating tumor DNA (ctDNA), fragments of DNA shed by tumor cells, provides a genetic snapshot of the tumor, enabling the identification of cancer-specific mutations. Highly sensitive techniques like droplet digital PCR (ddPCR) and next-generation sequencing (NGS) are employed to analyze ctDNA, offering varying levels of sensitivity and breadth. While ddPCR excels at detecting specific known mutations, NGS provides a broader view of the tumor's genomic landscape, including multiple mutations, copy number variations, and fusion genes. However, the low concentration of ctDNA in early-stage cancers can limit the sensitivity of these assays.
Circulating tumor cells (CTCs), intact cancer cells that have detached from the primary tumor and entered the bloodstream, offer another valuable source of information. Isolating and analyzing CTCs can provide insights into tumor heterogeneity, drug resistance mechanisms, and the tumor microenvironment. Techniques for isolating CTCs include size-based filtration, immunoaffinity methods that target tumor-specific surface markers, and density gradient centrifugation. Once isolated, CTCs can be analyzed for their genetic makeup, protein expression, and other cellular characteristics. However, the extreme rarity of CTCs in the blood presents a significant technical challenge for their efficient and reliable capture.
Exosomes, nano-sized vesicles released by cells, including tumor cells, represent an emerging area of focus in liquid biopsy. These vesicles carry a diverse cargo of proteins, nucleic acids, and lipids that reflect the cellular state of origin. Analyzing exosomes can provide valuable information about tumor progression, metastasis, and response to therapy. Techniques like mass spectrometry and advanced microscopy are employed to characterize the molecular contents of exosomes. However, the field of exosome analysis is still in its early stages, facing challenges related to standardization of isolation and analysis methods, as well as the complexity of interpreting the diverse molecular signals contained within exosomes.
The integration of multiple liquid biopsy approaches, often referred to as a multi-omics strategy, holds immense promise for enhancing the sensitivity and specificity of cancer detection and monitoring. Combining data from ctDNA, CTCs, and exosomes can provide a more comprehensive picture of the tumor's biology, revealing insights that may be missed by analyzing a single biomarker in isolation. This integrated approach, coupled with advancements in genomics and molecular biology, particularly next-generation sequencing (NGS), is driving the development of more sophisticated and informative liquid biopsy assays.
Liquid Biopsy in Cancer Care: Current and Future Applications
Liquid biopsy is demonstrating increasing clinical utility across various stages of cancer care. In early detection, studies using assays like CancerSEEK, which analyzes circulating tumor DNA, proteins, and other biomarkers, have shown promising results in detecting multiple cancer types with high specificity. For example, CancerSEEK demonstrated sensitivities ranging from 69% to 98% for several cancer types with a specificity of over 99%. While sensitivity for early-stage cancers remains a challenge, ongoing research is focused on improving the sensitivity of these assays and validating their performance in large-scale prospective trials.
Liquid biopsy offers a powerful tool for treatment monitoring and personalized medicine. By tracking changes in ctDNA levels and mutational profiles, clinicians can gain insights into tumor dynamics, treatment efficacy, and the emergence of drug resistance. For instance, a rebound in BRAF levels in ctDNA was observed in 60% of patients with progressive disease receiving BRAF-directed therapies. This real-time monitoring enables clinicians to adjust treatment strategies as needed, optimizing therapy for individual patients based on their tumor's molecular profile.
Liquid biopsy also plays a crucial role in monitoring minimal residual disease (MRD) and predicting cancer recurrence. After initial treatment, even when clinical imaging shows no signs of disease, microscopic cancer cells may persist, leading to relapse. Liquid biopsy can detect these residual cancer cells by analyzing ctDNA, providing a highly sensitive method for assessing MRD. Studies have shown that ctDNA-based MRD detection can predict recurrence months before it becomes clinically detectable, allowing for timely intervention and potentially improving long-term outcomes.
While promising, liquid biopsy faces challenges related to sensitivity, standardization, and cost. False negative results can occur, especially in early-stage cancers with low ctDNA concentrations. The lack of standardized protocols and the high cost of testing can also limit access and hinder widespread adoption. Furthermore, the interpretation of liquid biopsy results can be complex, requiring expertise in genomics and molecular oncology. Ongoing research aims to address these challenges and unlock the full clinical potential of liquid biopsy.
Investing in Liquid Biopsy: Opportunities and Challenges
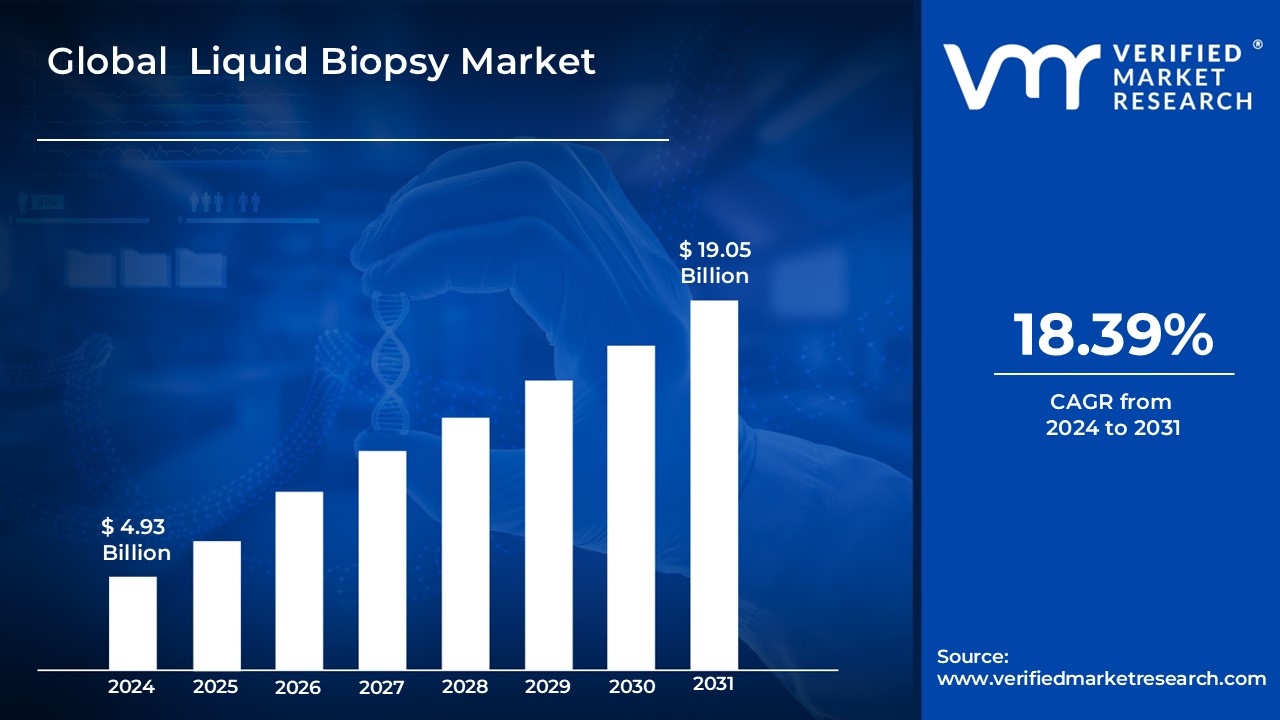
The liquid biopsy market is experiencing rapid growth, fueled by increasing clinical adoption and a surge in strategic acquisitions. Market reports estimate the global liquid biopsy market size to be between $4.93 billion and $5.39 billion in 2024, with projections reaching $19.05 billion to $20.41 billion by 2031 or 2033, representing a CAGR of 14.23% to 18.39%. This growth is driven by factors such as the rising incidence of cancer, the increasing demand for personalized medicine, and the advantages of liquid biopsy over traditional tissue biopsies. Several companies are attracting significant investment, including Grail, Freenome, and Thrive Earlier Detection, indicating strong investor confidence in the future of liquid biopsy.
Pharmaceutical companies are actively acquiring liquid biopsy companies to gain a competitive edge in this rapidly evolving field. Recent acquisitions include Veracyte's acquisition of C2i Genomics for $95 million, Exact Sciences' acquisition of Resolution Bioscience, and Quest Diagnostics' acquisition of Haystack Oncology for $450 million. These strategic moves aim to consolidate expertise, expand product portfolios, and integrate liquid biopsy into broader drug development and diagnostic strategies. However, regulatory hurdles, particularly FDA approval processes and reimbursement policies, remain significant challenges for market players.
The Future of Liquid Biopsy
Liquid biopsy holds immense promise for transforming cancer care, offering the potential for earlier detection, more personalized treatment, and improved patient outcomes. However, realizing this potential requires addressing key challenges such as improving sensitivity for early-stage cancers, standardizing testing protocols, and ensuring affordability and accessibility for all patients. Continued research, technological advancements, and collaboration among stakeholders will be crucial for driving innovation and shaping the future of liquid biopsy in the fight against cancer.
----------
Further Reads
I. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6024152/ctDNA and CTCs in Liquid Biopsy – Current Status and Where We Need to Progress - PMC
II. https://pubmed.ncbi.nlm.nih.gov/36096847/Liquid biopsy: current technology and clinical applications - PubMed
III. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11317461/Editorial: Liquid biopsy in oncology: opportunity and challenges - PMC